
NEWS : Process Engineer's Tools is moving to a new address, www.MyEngineeringTools.com, click here to discover the site |
Liquid Vapor Equilibrium : ideal mixtures
How to calculate the pressure and composition of phases of an ideal mixture ?
Follow us on Twitter ![]()
Question, remark ? Contact us at powder.process@protonmail.com
| Section summary |
|---|
| 1. Liquid Vapor
Equilibrium |
| 2. Composition of
Liquid and Vapor in equilibrium |
| 3. Non ideal mixtures |
1. Liquid Vapor Equilibrium
Example of a flash
The notion of liquid / vapor equilibrium is very important. In a situation of equilibrium, both liquid and vapor phases coexist, in a state of equilibrium, with the liquid and vapor saturated. The study of those equilibrium is very important for example for distillation.
At equilibrium, both the liquid phase and the vapor phase have the same pressure and the same temperature. Since the equilibrium is reached, the material exchange in between the liquid phase and the vapor phase is balanced : the same number of molecules leave the liquid phase to reach the vapor phase, as molecules from the vapor phase reach the liquid phase. The driving force for liquid molecules to reach the vapor phase is the saturation pressure of the liquid, which depends on the temperature, while the driving force for vapor molecules to go to the liquid phase is the pressure that is exerted.
Such situation of equilibrium especially happens in the case of a flash, which is the sudden partial vaporization of a liquid feed. This kind of separation happens in a so called flash vessel or flash drum which is maintained in certain conditions of pressure and temperature that allow coexistence and equilibrium in between 2 phases.
This kind of operation is very often found in refineries, petrochemical processes.

It is then required to perform an equilibrium calculation (also
called a flash calculation in this case) in order to calculate
the flows of liquid and vapor as well as the compositions.
Such equilibrium calculation can be more or less complicated
depending on the components of the mixture. The simpler case
presented in this page is an ideal mixture for which some
assumptions allowing to establish the well known Raoult Law are
possible.
2. Composition of liquid and vapor in equilibrium
2.1 Vapor composition at equilibrium (ideal mixtures) : Raoult's Law
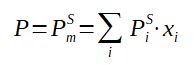
With
P = Pressure at equilibrium
PmS = Saturation pressure of the mixture
PiS = Saturation pressure of component i (note
: it is a function of the temperature)
xi = molar fraction of the component in the LIQUID phase
Validity
- Similar hydrocarbon mixtures
- Low pressure
It is assumed that the mixture is maintained at equilibrium, thus the pressure above the liquid is equal to the saturation pressure of the mixture. For ideal mixtures (hydrocarbon mixtures typically) it is possible to calculate the vapor pressure thanks to the composition of the liquid phase, this equation is called Raoult's law.
Not all mixtures are ideal and, when having in the mixtures components such as water (polar substances) a deviation to ideality will happen.
To go further in calculating the liquid / vapor equilibrium, it is then possible to introduce the Dalton law (Pi = yi.P), as the partial pressure of a component is equal to the total pressure times the molar fraction in the GAS phase.
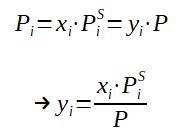
With
P = Pressure at equilibrium
PiS = Saturation pressure of component i
(note : it is a function of the temperature)
xi = molar fraction of the component in the LIQUID
phase
yi = molar fraction of the component in the GAS phase
2.2 Equilibrium coefficient
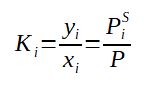
The application of the Raoult Law and Dalton Law leads to the expression of the ratio yi/xi which is called the equilibrium coefficient. The higher it is, the more the component will be volatile and will have the tendency to enrich the vapor.
It is important to note that Ki depends on the TEMPERATURE as the saturation pressure is only dependent on the temperature.
If Ki > 1 it means that the component has tendency to go in the vapor phase, it is volatile, when Ki < 1 it means that the component has tendency to stay in the liquid phase, it is heavy.
One more coefficient which is particularly interesting in distillation is the relative volatility which is comparing the equilibrium coefficient of 2 components.
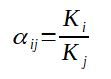
If α < 1, it means that the component i is heavier than the
component j
If α > 1, it means that the component i is lighter than the
component j, thus it will be possible to separate i from j through
distillation (succession of equilibrium liquid vapor in a column).
The higher α, the easier it will be.
To be noted that α increases when the pressure decreases, which explains why distillations are often done under vacuum in order to be more efficient.