
NEWS : Process Engineer's Tools is moving to a new address, www.MyEngineeringTools.com, click here to discover the site |
Flash Equilibrium Calculation of a mixture
How to calculate the pressure and composition of phases of a mixture of ≥ 2 components at equilibrium (flash calculation)
Follow us on Twitter ![]()
Question, remark ? Contact us at powder.process@protonmail.com
| Section summary |
|---|
| 1. Liquid Vapor
Equilibrium |
| 2. Calculation of
Liquid and Vapor in equilibrium |
1. Liquid Vapor Equilibrium
What needs to be calculated ?
When performing a liquid / vapor calculation, the characteristics
of the infeed are available (flow rate and composition) while the
characteristics of the vapor phase and liquid phase must be
calculated (flow rate and composition). For solving this calculation
it is also required to fix the conditions of the phases and / or
pressure and / or temperature depending on what needs to be
calculated.

For such a system, the following relations can be established
1.1 Material Balance
A = V + L
With
A = flowrate of infeed (mol/s, can be also kg/s if expressed in
mass fraction)
V = flowrate of vapor (mol/s or kg/s)
L = flowrate of liquid (mol/s or kg/s)
1.2 Balance on each component
A.zi = V.yi + L.xi
With
zi = molar or mass fraction of component i in the infeed
yi = molar or mass fraction of component i in the vapor
xi = molar or mass fraction of component i in the liquid
Also
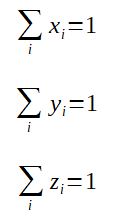
1.3 Equilibrium coefficient
Ki = yi/xi
yi = Ki.xi
With
Ki = equilibrium coefficient for component i
2. Calculation of liquid vapor equilibrium
2.1 Liquid at saturation and vessel at given pressure
In the 1st case studied, the infeed is purely liquid at its bubble point (which means it is just at saturation, but no phase change has happened yet, the infeed is only liquid). The Engineer wants to know what is the temperature corresponding to the bubble point.
zi = xi (no vapor phase)
yi = Ki.xi (general equilibrium expression) ⇒ yi = Ki.zi
Σ Ki.zi = 1
P is given
It is then necessary to have a model to calculate the equilibrium coefficient. It can be quite simple if the mixture is ideal (Ki = PiS / P) or more complex and require an equation of state. in any case, it is necessary to estimate the temperature to perform the calculations as Ki = f(T).
The following calculation procedure is then applied :
- Estimate the temperature T
- Calculate Ki = f(T)
- Calculate Σ Ki.zi
- If Σ Ki.zi = 1, T is correct and equal to the bubble temperature of the mixture
- If Σ Ki.zi ≠ 1, T needs to be recalculated by assuming a new value and going through the calculation procedure again
2.2 Vapor feed at saturation and vessel at given pressure
In the 2nd case studied, the infeed is purely gaseous at its dew point (which means it is just at saturation, but no phase change has happened yet, the infeed is only vapor). The Engineer wants to know what is the temperature corresponding to the dew point.
zi = yi (no liquid phase)
yi = Ki.xi (general equilibrium expression) -> zi = Ki.xi ⇒ xi = zi/Ki
Σ xi = 1
Σ zi/Ki = 1
P is given
It is then necessary to have a model to calculate the equilibrium coefficient. It can be quite simple if the mixture is ideal (Ki = PiS / P) or more complex and require an equation of state. in any case, it is necessary to estimate the temperature to perform the calculations as Ki = f(T).
The following calculation procedure is then applied :
- Estimate the temperature T
- Calculate Ki = f(T)
- Calculate Σ zi/Ki
- If Σ zi/Ki = 1, T is correct and equal to the bubble temperature of the mixture
- If Σ zi/Ki ≠ 1, T needs to be recalculated by assuming a new value and going through the calculation procedure again
2.3 Flash equilibrium calculation
In a flash drum, the conditions of the infeed are such that, when it arrives in the flash vessel at a lower pressure, it separates immediately in 2 phases in equilibrium.
A.zi = V.yi + L.xi (general mass balance)
yi = Ki.xi (equilibrium) -> A.zi = V.Ki.xi + L.xi⇒ xi = A.zi / (V.Ki + L)
Σ xi = 1 = Σ [A.zi / (V.Ki + L)]
P is given
An iterative calculation is then required
- Estimate the liquid flow L
- Calculate V = A - L
- Calculate Ki and Calculate xi
- If Σ xi = 1, L is correct and the flash calculation is done
- If Σ xi ≠ 1, a new value of L needs assumed and the calculation re-run
The method above works well when Ki = PiS/P
and therefore only depends on the pressure and temperature. If it is
not the case, then it is required to have another iteration loop
after step 3. After calculating xi and yi, the
coefficient Ki is recalculated. If it gives the same xi
as 1st calculated, then we can go to step 4, if not Ki is
calculated again with the new composition values until it converges.